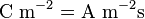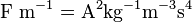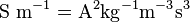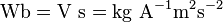Magnetism
Magnetism is a property of materials that respond to an applied
magnetic field.
Permanent magnets have persistent magnetic fields caused by
ferromagnetism.
That is the strongest and most familiar type of magnetism. However, all
materials are influenced varyingly by the presence of a magnetic field.
Some are attracted to a magnetic field (
paramagnetism); others are repulsed by a magnetic field (
diamagnetism); others have a much more complex relationship with an applied magnetic field (
spin glass behavior and
antiferromagnetism). Substances that are negligibly affected by magnetic fields are known as
non-magnetic substances. They include
copper,
aluminium,
gases, and
plastic. Pure
oxygen exhibits magnetic properties when cooled to a
liquid state.
An understanding of the relationship between
electricity and magnetism began in 1819 with work by
Hans Christian Oersted,
a professor at the University of Copenhagen, who discovered more or
less by accident that an electric current could influence a compass
needle. This landmark experiment is known as Oersted's Experiment.
Several other experiments followed, with
André-Marie Ampère,
who in 1820 discovered that the magnetic field circulating in a
closed-path was related to the current flowing through the perimeter of
the path;
Carl Friedrich Gauss;
Jean-Baptiste Biot and
Félix Savart, both of which in 1820 came up with the
Biot-Savart Law giving an equation for the magnetic field from a current-carrying wire;
Michael Faraday,
who in 1831 found that a time-varying magnetic flux through a loop of
wire induced a voltage, and others finding further links between
magnetism and electricity.
James Clerk Maxwell synthesized and expanded these insights into
Maxwell's equations, unifying electricity, magnetism, and
optics into the field of
electromagnetism. In 1905,
Einstein used these laws in motivating his theory of
special relativity,
[6] requiring that the laws held true in all
inertial reference frames.
SOURCE
- Electric currents or more generally, moving electric charges create magnetic fields (see Maxwell's Equations).
- Many particles have nonzero "intrinsic" (or "spin") magnetic moments. Just as each particle, by its nature, has a certain mass and charge, each has a certain magnetic moment, possibly zero.
It was found hundreds of years ago that certain materials have a
tendency to orient in a particular direction. For example ancient people
knew that "lodestones," when suspended from a string and allowed to
freely rotate, come to rest horizontally in the North-South direction.
Ancient Mariners used lodestones for navigational purposes.
In magnetic materials, sources of magnetization are the
electrons' orbital angular motion around the
nucleus, and the electrons' intrinsic magnetic moment (see
electron magnetic dipole moment). The other sources of magnetism are the
nuclear magnetic moments
of the nuclei in the material which are typically thousands of times
smaller than the electrons' magnetic moments, so they are negligible in
the context of the magnetization of materials. Nuclear magnetic moments
are important in other contexts, particularly in
nuclear magnetic resonance (NMR) and
magnetic resonance imaging (MRI).
Ordinarily, the enormous number of electrons in a material are
arranged such that their magnetic moments (both orbital and intrinsic)
cancel out. This is due, to some extent, to electrons combining into
pairs with opposite intrinsic magnetic moments as a result of the
Pauli exclusion principle (see
electron configuration), or combining into filled
subshells
with zero net orbital motion. In both cases, the electron arrangement
is so as to exactly cancel the magnetic moments from each electron.
Moreover, even when the
electron configuration is
such that there are unpaired electrons and/or non-filled subshells, it
is often the case that the various electrons in the solid will
contribute magnetic moments that point in different, random directions,
so that the material will not be magnetic.
Diamagnetism
Diamagnetism appears in all materials, and is the tendency of a material
to oppose an applied magnetic field, and therefore, to be repelled by a
magnetic field. However, in a material with paramagnetic properties
(that is, with a tendency to enhance an external magnetic field), the
paramagnetic behavior dominates.
[8]
Thus, despite its universal occurrence, diamagnetic behavior is
observed only in a purely diamagnetic material. In a diamagnetic
material, there are no unpaired electrons, so the intrinsic electron
magnetic moments cannot produce any bulk effect.
paramagnetism
In a paramagnetic material there are
unpaired electrons, i.e.
atomic or
molecular orbitals with exactly one electron in them. While paired electrons are required by the
Pauli exclusion principle
to have their intrinsic ('spin') magnetic moments pointing in opposite
directions, causing their magnetic fields to cancel out, an unpaired
electron is free to align its magnetic moment in any direction. When an
external magnetic field is applied, these magnetic moments will tend to
align themselves in the same direction as the applied field, thus
reinforcing it.
ferromagnetism
A ferromagnet, like a paramagnetic substance, has unpaired electrons. However, in
addition to the electrons' intrinsic magnetic moment's tendency to be parallel to
an applied field, there is also in these materials a tendency for these magnetic moments to orient parallel to
each other
to maintain a lowered-energy state. Thus, even when the applied field
is removed, the electrons in the material maintain a parallel
orientation.